Staying ahead of SO₂ emission limits with BASF’s high-performance catalyst, O4-116 Quattro


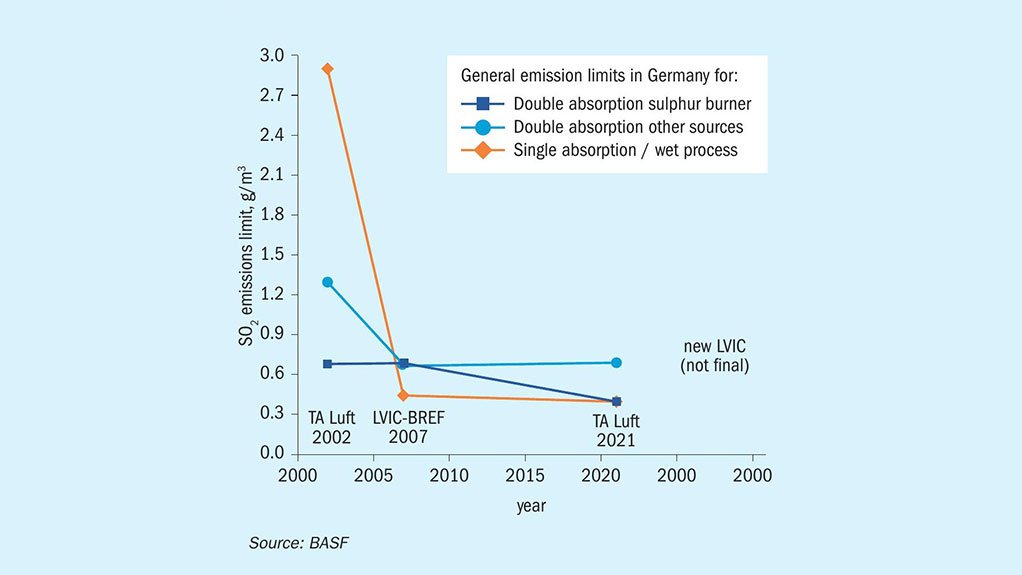
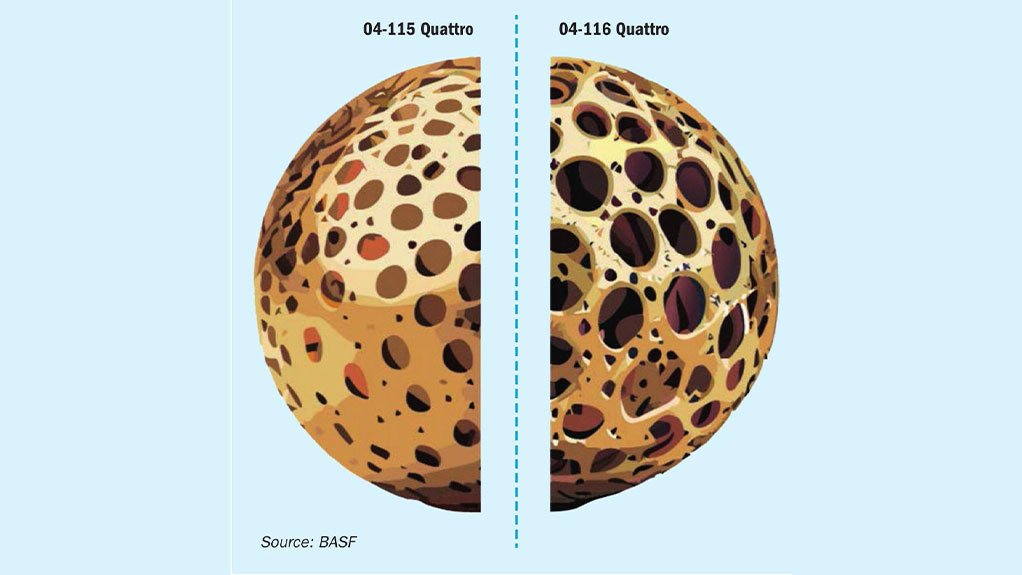
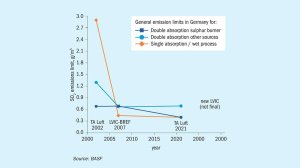
Fig. 1: Historic view on SO2 emission limits in Germany over the past 25 years. Special regulations for existing units with active permissions are not reflected here.
Fig. 2: Schematic comparison of the carrier material composition of O4-115 Quattro and O4-116 Quattro displaying a different pore structure.
By BASF SE Technology Manager for EMEA: Sulfuric Acid Catalysts Jochen Willersinn
Owing to ever stricter legislation towards lower SO2 emissions, superior catalyst shape and composition has become ever more important. Jochen Willersinn of BASF SE explains how BASF’s high-performance catalyst O4-116 Quattro combines the benefits of an increased surface area with a superior caesium-promoted active phase composition enabling significantly lower ignition and operating temperatures and thus reducing SO2 emissions.
BASF has produced sulphuric acid for various industrial applications since 1866 and has been producing catalyst for the sulphuric acid process since the early 20th century. Today, BASF operates six sulphuric acid and 12 sulphonation plants with inline SO2 oxidation units worldwide, all using BASF’s in-house catalyst technology with world class plants operating at emission levels below 50 ppm SO2. The last 20 years have brought new challenges such as tighter emission regulations and cost pressure to the sulphuric acid market. This has led BASF to be at the forefront with cutting-edge research into one of the oldest catalysts of the portfolio.
BASF works directly with customers to make sure customers achieve the best performance under the specific design and operation conditions of their reactors. This is enabled through BASF’s state-of-the-art testing facility and analytics combined with more than 150 years of research and experience.
SO2 Emission Regulation
Nearly all chemical and industrial processes result in a certain amount of by-products or waste components, which are harmful for our environment. The impacts are plentiful and can lead to soil contamination, eutrophication and, most importantly, to global warming. To reduce their negative effects on the environment, the emission of these harmful components is regulated and measures are taken by companies to reduce their emissions. Sulphur dioxide (SO2) and sulphur trioxide (SO3) also fall under these regulations. Starting from the first ratification of the Helsinki Protocol in 1985, the emission limits were further decreased, and other protocols were implemented to decrease the amount of SOx emitted through various sources (e.g. automotive, electricity generation and industrial processes) namely the Oslo Protocol in 1994 and the Gothenburg Protocol in 1999, which was further amended in 2012. Since SO2 is not just poisonous, but also a major contributor to acid rain when it is released to the atmosphere, the emission limits for SO2 for sulphuric acid plants became ever stricter over the past decades. The graph in Fig. 1 displays the development for average SO2 emission limits for sulphuric acid producing units in Germany, which follow the regulations provided by the European Union and the German Federal Government.
As displayed in Fig. 1 (See on the image slides above), there has been a significant decrease in emission limits for the most common sulphuric acid producing technologies over the past 25 years in Germany. Further, a new LVIC-BREF (best available techniques reference document for the manufacture of large volume inorganic chemicals) for the EU is under discussion which will most likely result in a further decrease in SO2 emission limits in the near future. These emission limit reductions for SO2 resulted in several changes in the industry: The use of scrubbers to remove excess SO2 for single contact units, double contact units with five catalyst beds, and the implementation of caesium-promoted catalysts that are active at lower temperatures to maximise the conversion of SO2 to SO3. Among others, these changes made a major contribution to the reduction of SO2 emissions whilst also maximising the sulphuric acid output of these units. However, as stricter emission regulations are already on the horizon, more powerful tools need to be implemented in the future to be able to comply with the regulations.
Fig. 1: Historic view on SO2 emission limits in Germany over the past 25 years. Special regulations for existing units with active permissions are not reflected here.
New Extruded Shapes
An elegant way to accomplish this is with the use of even more powerful catalysts, which enable higher conversion rates. BASF’s approach to achieve this is through the improvement of catalyst shape geometry.
In 2016 BASF launched the Quattro shape geometry for its sulphuric acid catalyst family. This outstanding catalyst shape geometry combines a 30% increased surface area with significantly increased catalyst strength whilst maintaining the pressure drop of Star Ring catalysts.
Since the launch of O4-115 Quattro in 2016, many additional customers are benefiting from realised performance improvements, such as higher capacity, lower catalyst loading volume, or significant conversion increase. A major gamechanger in emission reduction is BASFs high performance catalyst O4-116 Quattro. This catalyst combines the geometrical benefits of the Quattro family with a superior active mass composition and enables significant reduction in emissions.
O4-115 versus O4-116
For caesium-promoted catalysts, there are two different application fields. The first application field is as an ignition layer for the first pass. This is mostly necessary in metallurgical plants, where the inlet temperature to the first bed can fluctuate and is in most cases around 400 °C. The second field of application is for emission reduction after the intermediate absorption step as described above. As both fields have a different set of requirements, such as different upper operating temperatures, or mass transfer properties, it is not favourable to use one single catalyst to do both jobs, as a lot of potential remains unused. Hence, BASF developed two different caesium-promoted catalysts in its portfolio, namely O4-115 as the catalyst dedicated to operating in bed 1 ignition layers and in single absorption units, i.e. situations with high SO2 and SO3 concentrations where the conversion is mostly equilibrium controlled. O4-116 was developed as the high-performance low temperature catalyst for applications after the intermediate absorption, where a high mass transfer rate is key to maximise the conversion.
These catalysts have a different amount of caesium in their composition, which is regulating the melting point of the active phase and therefore the activity at low temperatures. But that is only a small part of what distinguishes these catalysts. The choice of the carrier material composition (Fig. 2) is a crucial step for the desired application field of a catalyst.
Fig. 2: Schematic comparison of the carrier material composition of O4-115 Quattro and O4-116 Quattro displaying a different pore structure. (See on the image slides above)
As displayed in the schematic in Fig. 2, the key difference between O4-115 and O4-116 is the pore structure and pore size distribution of those catalysts. The carrier material of O4-115 is specifically designed to operate under conditions where the oxidation reaction of SO2 to SO3 is equilibrium controlled meaning high temperatures and high SO2 and SO3 concentrations. Here, the stability of the catalyst is crucial, to withstand the extremes it is experiencing and an increase in porosity has no apparent benefit, as the reaction is not mass transfer limited. The opposite is the case for the intended application field of O4-116. Here mass transfer limitation is the key issue, which needs to be overcome by a superior pore size structure to enable a fast diffusion of all reactants in and out of the active phase.
Surpassing State-of-the-Art Performance
When applying the fundamental concepts of a change in shape geometry from Star Ring to Quattro, O4-116 displays not just the same benefits as seen with the other catalysts, such as a higher mechanical strength, lower attrition and a higher geometric surface area. The activity increase surpasses the expected boost originating from the higher geometric surface area. This is a result of a synergistic effect between the pore structure of the O-116 carrier material and the Quattro shape geometry surpassing the activity standard catalysts by far. When comparing the kinetic rates of O4-115 Quattro and O4-116 Quattro, O4-116 Quattro has a significantly higher activity between 20% and 40% at temperatures below 405 °C. This is significantly higher than is the case for the Star Ring geometries and can only be explained by a synergistic effect, since the base composition of the catalyst is not changed and only a change in the shape geometry was conducted.
BASF has data and references. Get it all from Dr. Saul Colley | Africa Sales & Technical Support, Catalysts and Adsorbents: saul.colley@basf.com
Learn more about BASF’s high-performance Sulphuric Acid catalyst solutions here:
Article Enquiry
Email Article
Save Article
Feedback
To advertise email advertising@creamermedia.co.za or click here
Announcements
What's On
Subscribe to improve your user experience...
Option 1 (equivalent of R125 a month):
Receive a weekly copy of Creamer Media's Engineering News & Mining Weekly magazine
(print copy for those in South Africa and e-magazine for those outside of South Africa)
Receive daily email newsletters
Access to full search results
Access archive of magazine back copies
Access to Projects in Progress
Access to ONE Research Report of your choice in PDF format
Option 2 (equivalent of R375 a month):
All benefits from Option 1
PLUS
Access to Creamer Media's Research Channel Africa for ALL Research Reports, in PDF format, on various industrial and mining sectors
including Electricity; Water; Energy Transition; Hydrogen; Roads, Rail and Ports; Coal; Gold; Platinum; Battery Metals; etc.
Already a subscriber?
Forgotten your password?
Receive weekly copy of Creamer Media's Engineering News & Mining Weekly magazine (print copy for those in South Africa and e-magazine for those outside of South Africa)
➕
Recieve daily email newsletters
➕
Access to full search results
➕
Access archive of magazine back copies
➕
Access to Projects in Progress
➕
Access to ONE Research Report of your choice in PDF format
RESEARCH CHANNEL AFRICA
R4500 (equivalent of R375 a month)
SUBSCRIBEAll benefits from Option 1
➕
Access to Creamer Media's Research Channel Africa for ALL Research Reports on various industrial and mining sectors, in PDF format, including on:
Electricity
➕
Water
➕
Energy Transition
➕
Hydrogen
➕
Roads, Rail and Ports
➕
Coal
➕
Gold
➕
Platinum
➕
Battery Metals
➕
etc.
Receive all benefits from Option 1 or Option 2 delivered to numerous people at your company
➕
Multiple User names and Passwords for simultaneous log-ins
➕
Intranet integration access to all in your organisation